Aravalli Green Wall Project (GS Paper 3, Environment)
Why in news?
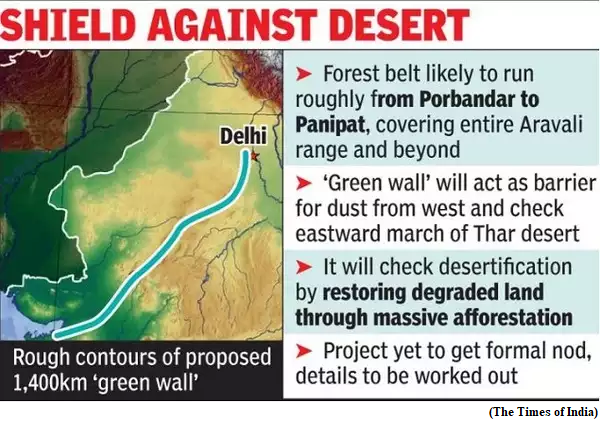
- Recently, the Union Minister for Environment, Forest and Climate Change, launched the Aravalli Green Wall Project, a major initiative to green the 5 km buffer area around the Aravalli Hill Range in four states at a function organised to celebrate the International Day of Forests at Tikli Village in Haryana.
- He also unveiled the ‘National Action Plan to Combat Desertification and Land Degradation through Forestry Interventions’ and a FAQ on Agro-forestry published by Indian Council of Forestry Research and Education.
Key Highlights:
- In the initial phase, 75 water bodies will be rejuvenated under the project, starting with five waterbodies each in every district of Aravalli landscape on March 25th.
- The project will also include large-scale plantation drives and conservation of water resources in the Aravalli region.
- The project will cover degraded land in Gurgaon, Faridabad, Bhiwani, Mahendergarh and in Rewari districts of Haryana.
- Voluntary organization, Society for Geo informatics and Sustainable Development and NGO, IMGurgaon are being engaged to mobilize people for Shramdaan for revival of water bodies at Bandhwadi and Ghata Bundh respectively.
The Aravalli Green Wall Project has the following objectives:
- Improving the ecological health of the Aravalli range
- To prevent eastward expansion of Thar Desert and to reduce land degradation by creating green barriers that will prevent soil erosion, desertification and dust storms
- This green wall will help in carbon sequestration and mitigating climate change to enhance the biodiversity and ecosystem services of the Aravalli range by planting native tree species in the Aravalli region, providing habitat for wildlife, improving water quality and quantity.
- Promote sustainable development and livelihood opportunities by involving local communities in afforestation, agro-forestry and water conservation activities that will generate income, employment, food security and social benefits.
- The project will be executed by various stakeholders such as central and state governments, forest departments, research institutes, civil society organisations, private sector entities and local communities. Adequate funding, technical skills, policy coordination and public awareness will be called upon to ensure the success of the project.
- Contribute to India's commitments under various international conventions such as UNCCD (United Nations Convention to Combat Desertification), CBD (Convention on Biological Diversity) and UNFCCC (United Nations Framework Convention on Climate Change).
- Enhancing India's image as a global leader in environmental protection and green development.
About Aravalli Green Wall Project:
- The Aravalli Green Wall Project is part of the Union Environment Ministry's vision to create green corridors across the country to combat land degradation and desertification.
- The project covers states of Haryana, Rajasthan, Gujarat and Delhi - where the Aravalli hills landscape span over 6 million hectares of land.
- The project will involve planting native species of trees and shrubs on scrubland, wasteland and degraded forest land, along with rejuvenating and restoring surface water bodies such as ponds, lakes and streams.
- The project will also focus on agroforestry and pasture development to enhance the livelihoods of local communities.
Why did India reject J&J’s patent on TB drug?
(GS Paper 2, Health)
Why in news?
- Recently, the Indian Patent Office rejected an application by pharmaceutical giant Johnson & Johnson (J&J) to extend its patent on the drug bedaquiline beyond July 2023.
- Bedaquiline is a drug in tablet form used to treat drug-resistant tuberculosis (TB).
- This opens the door for drug manufacturers to produce generic versions of bedaquiline, which are expected to be more affordable and to contribute to India’s goal of eliminating TB by 2025.

What is drug-resistant TB?
- As of 2017, India accounted for around one-fourth of the world’s burden of multi-drug-resistant (MDR) TB and of extensively-drug-resistant (XDR) TB.
- MDR TB resists treatment by at least two frontline drugs in TB treatment, isoniazid and rifampicin.
- XDR TB resists these two drugs as well as fluoroquinolones and any second-line injectable drug. XDR TB is rarer than MDR TB — there were 1,24,000 cases of the latter in India (2021) versus 2,650 cases of the former (2017).
- TB incidence in India has been on the decline, but MDR TB and XDR TB endanger initiatives to locally eradicate the disease. In the first two years of the pandemic, there were reports that TB treatment was hit by disrupted supply chains, availability of healthcare workers for non-pandemic work, and access to drug-distribution centres.
How is drug-resistant TB treated?
- TB is an infection of the bacterium Mycobacterium tuberculosis in the lungs, but often in other organs as well.
- It can be treated by strictly adhering to the doses and frequencies of drugs prescribed by a physician. Deviations from this schedule can lead the bacteria to become drug-resistant. Yet they happen because the drugs often have side effects that diminish the quality of life and/or because patients haven’t been afforded access to the requisite drugs on time.
- Drug-resistant TB is harder to treat. One important option for those diagnosed with pulmonary MDR TB is bedaquiline.
- In 2018, the World Health Organization replaced two injectable drugs for MDR TB with an oral regimen that included bedaquiline.
How effective is bedaquiline?
- Typically, bedaquiline needs to be taken for six months: at a higher dose in the first two weeks followed by a lower dosage for 22 weeks. This period is shorter than other treatment routines for pulmonary MDR TB, which can last 9-24 months.
- One phase II clinical trial observed that culture conversion (turning a patient’s sputum culture from positive to negative) “at 24 weeks was durable and associated with a high likelihood of response at 120 weeks”, due to bedaquiline.
- Unlike second-line treatment options that are injected and can have severe side effects, like hearing loss, bedaquiline is available as tablets and is less harmful, although it has potential side effects of its own.
Why was the patent application rejected?
- J&J’s patent application was for a fumarate salt of a compound to produce bedaquiline tablets. Two groups opposed the patent: 1) Network of Maharashtra people living with HIV and 2) Nandita Venkatesan and Phumeza Tisile, both TB survivors, supported by Médecins Sans Frontières.
- Both groups argued that J&J’s method to produce a “solid pharmaceutical composition” of bedaquiline is “obvious, known in the art” and doesn’t require an “inventive step”.
- According to the Indian Patent Act 1970 Section 2(1)(ja), an ‘inventive step’ is an invention that is “not obvious to a person skilled in the art”.
- The latter also contended that the current application drew significantly from a previous patent, WO 2004/011436, which discussed a similar compound on which bedaquiline is based and whose priority date (2002) well preceded the new application.
- The Patent Office rejected the application on these and other grounds, including Sections 3d and 3e of the Act.
- These pertain to “mere discovery of a new form of a known substance which does not result in the enhancement of the known efficacy of that substance” and “a substance obtained by a mere admixture resulting only in the aggregation of the properties of the components thereof”, respectively, which are not patentable.
Why is the rejection notable?
- India has the largest population of people living with drug-resistant TB. J&J’s patent on bedaquiline meant the drug cost $400 (revised to $340 in 2020) per person, plus the cost of other drugs. The rejection is expected to lower the cost of bedaquiline by up to 80%.
- So far, the Indian government has directly procured the drug and distributed it through State-level TB programmes. After July 2023, manufacturers of generic drugs such as Lupin will be able to produce generic versions of bedaquiline.



